For the first time, an experimental treatment made from human embryonic stem cells has shown evidence of helping someone, partially restoring sight to two people suffering from slowly progressing forms of blindness
By Rob Stein and David Brown
For the first time, an experimental treatment made from human embryonic stem cells has shown evidence of helping someone, partially restoring sight to two people suffering from slowly progressing forms of blindness.
Although the purpose of the experiment was to test the safety of stem cells injected into the eye, both patients “had measurable improvement in their vision that persisted through the duration of the study,” said Robert Lanza, chief scientific officer at Advanced Cell Technology, the Massachusetts biotech company that sponsored the closely watched experiment.
The operations in July on two Southern California women yielded practical results. One of them no longer needs a large magnifying glass to read and can reportedly thread a needle. The other has begun to go shopping on her own.
Reported online in the Lancet on Monday, the project used the cells under highly favorable conditions not likely to exist with many diseases.
The cells were transplanted into the eye, an organ in which the chance of immune rejection is low. The complex, 10-layer architecture of the retina was intact, so the cells were not asked to perform a heroic act of reconstruction. The researchers were able to monitor progress — and watch for complications — in real time by looking into the eyes.
Lanza cautioned that the findings are preliminary, the improvements could disappear and complications could emerge. Nevertheless, he thinks the two cases will provide useful lessons for the field.
“Hopefully, this is just the beginning of many exciting new stem cell therapies that will move from bench to bedside in the next few years,” he said.
This field of research has stirred high hopes and bitter debate in recent years.
Embryonic stem cells are able to develop into virtually any type of tissue in the body, but to obtain them, embryos are manipulated and sometimes destroyed. Many researchers hope the cells — or other “pluripotent” cells derived from less controversial sources — will offer cures for heart disease, cancer, Alzheimer’s dementia, spinal cord injury and other ailments.
Until now, the strategy had shown promise only in laboratory studies and animal tests. After many delays, the Food and Drug Administration last year approved two experiments in people. The blindness trial is the first to publish evidence that the approach might be working.
“We have to be careful not to turn this into: ‘Everybody needs stem cells right now,’” said Steven D. Schwartz, an ophthalmologist at UCLA’s Jules Stein Eye Institute who is leading the experiment. “But what it does say is that in the short term, this has been surprisingly positive.”
The report was not greeted with enthusiasm everywhere.
“We object to the idea that you would sacrifice some members of the human race, even at the earliest stage of development, for the potential treatment of other members of the human race,” said David Prentice, a cell biologist at the Family Research Council. He said he thinks other strategies, such as one that uses skin cells that have been “induced” to become stem cells, are more promising and ethical.
This is not the first time embryonic stem cells have been injected into people for therapeutic purposes.
In October 2010, doctors in Atlanta injected millions of cells into the spine of a 21-year-old Alabama nursing student partially paralyzed by a car accident. Subsequently, four other similar patients have been treated. The only results released about those patients is that none has suffered any harm. But Geron, which was sponsoring the study, recently announced that money problems had forced the company to abandon plans to treat more patients, stunning researchers, advocates and patients.
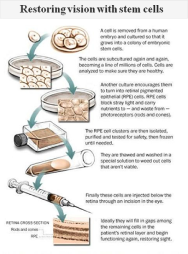
In the new study, each patient received about 50,000 “retinal pigment epithelium” cells descended from a single cell taken years ago from an eight-cell embryo. Obtaining the cell did not destroy the embryo — which was left over from a test-tube fertilization procedure at a clinic in New England — but the embryo was subsequently destroyed.
One patient in the new study is a 51-year-old graphics artist from Los Angeles, and the other is a 78-year-old woman from Laguna Beach, Calif. Both had some vision but were legally blind. In interviews with The Washington Post weeks before the results were made public, both patients said they were thrilled.
“It’s a great gift, really,” said the Los Angeles woman, who spoke on the condition of anonymity to protect her privacy. “I’m ecstatic. It’s a big surprise.”
Within about six weeks of having the cells infused into her eye, the Los Angeles graphic artist noticed something.
“I just woke up one morning and looked through one eye and the other, and the difference was pretty dramatic,” she said. “I have an armoire across my room, and it has a lot of carved detail on it. I looked at it with the eye they operated on and could see all that detail. I just wanted to look at everything. It’s sort of like having new eyes.”
Since then, her vision has improved, enabling her to read more characters on an eye chart, thread a needle and see colors, she said.
“There’s more sharpness and contrast. I recognize people better. I see their faces better,” she said.
Her sight had started fading when she was in her 20s because of Stargardt’s macular dystrophy, a progressive form of blindness that often begins in childhood. She lost most of the vision in the center of each eye. Working became increasingly difficult. Recognizing even familiar faces and watching television soon went. Since the July 12 procedure, she’s regained enough vision to start biking again.
Tests indicate that healthy cells have grown where the treatment was injected, increasing confidence that the cells are responsible for her better sight.
“She’s got some visual improvement we can document and that plausibly corresponds to the transplant. We see the cells engrafting and thriving in areas that correspond to her improved vision, which is remarkable and unexpected at this point,” Schwartz said.
The second patient, Sue Freeman, had been slowly losing her vision for more than 20 years because of dry macular degeneration, the leading cause of blindness in the developed world. Freeman had been forced to stop driving. It became impossible to recognize friends and family members’ faces. Soon, she stopped venturing out alone.
“I quit going to the grocery store because I couldn’t see any of the labels. I dropped out of organizations I was in. I couldn’t take a job because I couldn’t read. It definitely changed my life,” Freeman said. “It kind of shut me down.”
But within about six weeks of the procedure, Freeman started noticing changes.
“I started telling my husband, ‘You know, things seem brighter to me.’ I don’t know if it’s my imagination, but looking at landscapes seemed brighter,” she said, noting that she persuaded her husband to drop her off at a mall by herself for the first time in years.
“He was a wreck, but it was fine. I thought: ‘Oh my God. I did this on my own,’” she said, noting that she had also begun to read, cook for herself and use a watch.
The researchers initially weren’t sure whether her improvement was attributable to the operation or a placebo effect. They couldn’t see clear evidence of new cells in their examination, and she said vision in both eyes was better. Further testing, however, has convinced the team that Freeman’s sight is better.
After growing large numbers of stem cells, the researchers coaxed them to differentiate into nearly full-grown retinal pigmented epithelium cells. The latter do not themselves detect light, but without them, the photoreceptor cells in the retina cannot survive.
Both patients were on immunosuppressive drugs for about 12 weeks. They are now off them, and their eyes are without evidence of inflammation or tissue rejection, Lanza said.
The money for the experiment was provided by investors in Advanced Cell Technology; there were no government or foundation research grants, Lanza said.
The research team has permission to treat 12 patients with dry macular degeneration and 12 with Stargardt’s macular dystrophy. A second patient with the latter disease got a stem cell transplant in London on Friday. The team will try four different doses of stem cells — from 50,000 to as many as 200,000 cells — in groups of three patients each. The goal is to try the treatment in an early stage of the disease to prevent all vision loss.
View article at Washingtonpost.com